- Home
- The Mind
- Issue 2023/1
- Issue 2023/2
- Issue 2023/3
- Issue 2024/1
- Issue 2024/2
- Issue 2024/3
- Editorial
- Commentary
- Abstract 1
- Abstract 2
- Abstract 3
- Abstract 4
- Abstract 5
- Abstract 6
- Abstract 7
- Abstract 8
- Abstract 9
- Abstract 10
- Abstract 11
- Abstract 12
- Abstract 13
- Abstract 14
- Abstract 15
- Abstract 16
- Abstract 17
- Abstract 18
- Abstract 19
- Abstract 20
- Abstract 21
- Abstract 22
- Abstract 23
- Abstract 24
- Abstract 25
- Abstract 26
- Abstract 27
- Abstract 28
- Issue 2025/1
- Issue 2025/2
- Issue 2025/3
- Contact
- MBMRC
- Events
Commentary
The Impact of Inflammation and Nutrition on Attention-Deficit/Hyperactivity Disorder (ADHD): Potential Contributions to its Development and Symptoms
by Simon Weissenberger1
1Department of Psychology, University of New York in Prague, Prague, Czechia, 120 00
Cite as: Weissenberger, S. (2025). The Impact of Inflammation and Nutrition on Attention-Deficit/Hyperactivity Disorder (ADHD): Potential Contributions to its Development and Symptoms. THE MIND Bulletin on Mind-Body Medicine Research, 7, 16-23. https://doi.org/10.61936/themind/202504305
Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a complex neurodevelopmental disorder characterized by inattention, hyperactivity, and impulsivity. Emerging evidence links ADHD to biological factors such as neuroinflammation, oxidative stress, and mitochondrial dysfunction. Environmental exposures, nutritional imbalances, and genetic composition have a profound influence on these factors. Recent research highlights the contributions of inflammatory markers, hormonal dysregulation, and maternal immune activation to the etiology of ADHD. Given that deficiencies in omega-3 fatty acids and antioxidants can exacerbate symptoms, interventions such as anti-inflammatory diets and dietary supplementation have shown promise. Recent literature highlights the importance of these insights.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a widespread neurodevelopmental disorder that is estimated to afflict approximately 5.29% of the world’s population (Polanczyk et al., 2007). ADHD is associated with a wide array of symptoms and comorbidities. The most common of these symptoms are inattention, restlessness, behavioral issues such as impulsivity, and often overlooked affective issues such as emotional dysregulation (American Psychiatric Association, 2013). Along with the classical symptoms that are included in the diagnostic criteria for ADHD, this disorder also encompasses a wide variety of comorbidities and negative health outcomes, including obesity, proneness to accidents, addictions, and sleep issues. These comorbidities develop primarily in patients diagnosed with ADHD who have not undergone drug (e.g., stimulant) treatment and psychotherapy (Nigg, 2013). Given the combination of attention-related and affective issues together with the aforementioned comorbidities, ADHD can be a very detrimental condition and lead to a wide variety of negative health outcomes. The importance of appropriate treatment and management of the disorder should not be underestimated if we hope to prevent a wide array of negative health and quality of life outcomes. A variety of biological aspects have also been associated with ADHD. Oxidative stress and high levels of systemic inflammation have been identified as key components of ADHD; antioxidant compounds including plant polyphenols may mitigate these symptoms and have been proposed as adjunct therapies to conventional treatments (Varlaet et al., 2018). In recent years, we have gained a more comprehensive understanding of biological inflammation secondary to toxin exposure and viral infections and its impact on mental health (Esch & Stefano, 2002; Esch et al., 2002). Importantly, neuroinflammation secondary to viral infections and subsequent lingering effects of several virus-associated diseases may also contribute to the exacerbation of psychiatric symptoms. Prenatal exposure to infections and toxins has also been identified as an important risk factor for disorders such as ADHD (Büttiker et al., 2022; Freitas et al., 2020, Stefano et al., 2024; Freitas et al., 2020; Büttiker et al., 2024; Stefano, 2024).
Many individuals diagnosed with ADHD also present with dietary problems, including imbalances in omega-3:6:9 fatty acid ratios and critical mineral deficiencies; these issues have also been identified as potential risk factors for expectant mothers and their as-yet-unborn children (Sinn, 2008). Collectively, these disorders lead to a cycle of ongoing inflammation and continued exacerbation of psychiatric symptoms, suggesting that a more holistic take on mental health is warranted.
2. Recent Studies: ADHD and Inflammation
Etiologically speaking, psychiatric disorders develop in response to a series of complex interactions among factors that are regulated by genetics, epigenetics, and the environment. Further insight into a wide variety of mental health conditions can be obtained by focusing on the interactions between the immune system and the gut microbiome and taking into account the prominent roles played by neuroinflammation and other inflammatory responses (Alam et al., 2017, Stefano et al., 2018). The potential impact of circulating cytokines and inflammatory responses on those diagnosed with ADHD has been recognized for more than a decade. For example, the results of a recent study identified neuroinflammation as a key pathogenetic factor in an animal model of ADHD that was accompanied by elevated rates of autoimmune disease and higher levels of cytokines in the peripheral circulation (Dunn et al., 2019). Many factors are known to contribute to these neuroinflammatory responses, including viral infections (e.g., coronavirus disease 2019 [COVID-19]) (Büttiker, et al. 2022) and heavy metal toxins (e.g., mercury, lead) that have been detected at higher levels in individuals diagnosed with ADHD compared to those who were not (Tabatazde et al., 2018). Other reviews of recent literature reported that the COVID-19 pandemic and post-COVID syndrome had a clear negative impact on those diagnosed with attention disorders (Lopez-Leon et al., 2021). Interestingly, Oades et al. (2010) reported a clear difference in the pro-inflammatory markers found among those with ADHD who were medicated compared to those who were drug-naïve, with diminished levels of proinflammatory cytokines and other inflammatory markers observed among those in the medicated group. Most viral infections (e.g., COVID-19) are also associated with higher levels of circulating pro-inflammatory cytokines (Anka et al., 2021).
Maternal immune responses and their subsequent effects in utero along with specific toxin exposure (e.g., Bisphenol-A and other plasticizers) have also been associated with subsequent neuroinflammatory responses, ADHD, and a variety of other disorders (Han et al, 2021; Weissenberger et al., 2017). In their 2020 experiment on Taiwanese children diagnosed with ADHD, Chang et al. (2020) identified several differences between the ADHD group and neurotypical children, most notably higher levels of inflammatory biomarkers such as cytokines and neurotrophins and lower levels of salivary cortisol measured at various time points throughout the day. Some researchers have suggested that levels of pro-inflammatory interleukins, cortisol, and other immune cells might be developed for use as potential biomarkers for ADHD (Park, 2022). Other reviews of the literature reported that although the evidence for their use as definitive biomarkers remains inconclusive, they do state unequivocally that inflammation and paradoxically lower cortisol levels remain key components of the ADHD phenotype (Leffa et al., 2018). Results from other studies revealed an association between inflammatory skin conditions (e.g., atopic eczema) and current as well as future ADHD status in children (Kim et al., et al., 2020). Furthermore, a review of studies focused on the contributions of the endocannabinoid system (ECS) elucidated the impact of endocannabinoid receptor imbalances in a variety of mental health conditions, including ADHD, associated with positive allostasis of the cannabinoid receptor 1 (CB1). The authors also suggested possible endocannabinoid-associated biomarkers for a variety of conditions (Navarrette et al., 2020). As research in this field develops further, the association of ECS with disorders such as ADHD may develop into a path toward critical future treatments (Katzman et al., 2016).
Some researchers have suggested a role for nutritional supplementation in ADHD, including zinc, magnesium, and most importantly, omega-3 fatty acids found in fish oils or algae extracts. While some evidence suggests that omega-3 supplements and other minerals can improve ADHD symptoms in children (Bloch & Mulqueen, 2014), others reported that the data on docosahexaenoic acid and fatty acid supplementation remains unconvincing, especially in studies of adults with this disorder (Bonvicini et al., 2016).
Interestingly, supplementation with polyunsaturated fatty acids and fats with high levels of omega-3 and omega-9 compared to omega-6 fatty acids may be highly effective at reducing systemic inflammatory responses. Western diets often create fatty acid imbalances as they include foods with very high omega-6 to omega-3 ratios. Omega-6 fatty acids are the precursors to arachidonic acid, which is itself a precursor to the eicosanoids that contribute to elevated cellular inflammatory responses. Maintaining an appropriate dietary omega-3:6 balance has been recognized as beneficial for other psychiatric conditions, notably depression, which is also known to include an inflammatory component (Freeman & Rapaport, 2011). The cannabinoid receptor CB1 has also been linked to ADHD along with more classically-recognized dopamine receptor D4 gene mutations, with the two possibly being connected (Navarete et al., 2020). Metabolic syndrome and obesity are now also recognized as signs of ECS imbalances; maternal obesity and associated inflammatory responses have also been identified as major risk factors for the future development of ADHD (Sanchez et al., 2018; van der Burg et al., 2016). Meanwhile, dietary supplementation with healthy fats also tends to improve the efficacy of stimulant medications prescribed for children (Millichap & Yee, 2012).
3. Diet and Antioxidants
A variety of nutritional deficiencies in expectant mothers have been identified as risk factors for the development of ADHD in their children. These nutrient deficiencies include zinc, magnesium, and iodine along with inadequate intake of healthy fats (Konkiowska et al., 2012). In 2017, Rios-Hernandez and colleagues (2017) conducted a study on 120 children and adolescents diagnosed with ADHD and found that symptom intensity correlated with lower adherence to a Mediterranean-type diet (high in fruits, vegetables, and healthy fats with minimal red meat) and a much higher self-reported intake of “junk food” (i.e., food that is ultra-processed and contains high levels of unhealthy fats, and high sugar content) and higher rates of soda consumption. Results from another similar study revealed that adolescents with ADHD exhibited lower adherence to a Mediterranean-style diet and higher consumption of “junk food” (Khazdouz et al., 2024). Similarly, in our 2018 study that included a representative sample of the Czech adult population, we found that higher intensity ADHD symptoms were positively correlated with poorer diets, including those that consisted primarily of “fast food” along with higher consumption of sweets and ultra-processed foods; interestingly, the intensity of ADHD symptomatology in this study was also inversely correlated with physical exercise and nicotine intake (Weissenberger et al., 2018). Given that elevated levels of inflammatory markers are typically found at baseline in children diagnosed with ADHD, the rationale for including antioxidant-rich foods and possibly antioxidant supplementation appears to be sound (Verlaet et al, 2018). Certain compounds, including the amino acid and mucolytic N-acetyl cysteine (NAC), have been administered safely in this population and appear to help with several psychiatric conditions, including drug addiction and ADHD. Of note, NAC serves as a glutathione precursor and can thus be considered an antioxidant (Deepmala et al., 2015; Aldini et al., 2018). Recently, Wang and Qi (2022) reported that the carotenoid anti-oxidant, astaxanthin, which crosses the blood-brain barrier, might be used to limit neuroinflammation. Likewise, in a meta-review on cannabinoids and their potential use to promote mental health, the non-psychoactive and neuroprotective agent, cannabidiol was found to be moderately effective in treating ADHD symptoms (Khan et al., 2020). Antioxidant supplementation in addition to a balanced diet should also be taken into consideration because, in general, children with ADHD seem to suffer from deficiencies in key minerals and vitamins, including magnesium, zinc, selenium, and the B-vitamins (Dura Trave, et al. 2014). An overview of the interplay between pathogenic sequelae, along with nutrition as either a pro-inflammatory contributor to symptomatology or a protective factor is illustrated in Figure 1.
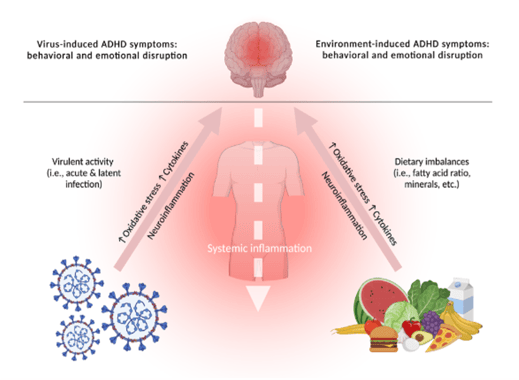
Fig. 1. The dynamic interplay between pathogens, inflammation, and nutrition in the human organism.
4. Cellular Energy Metabolism, Mitochondria, and ADHD
Mitochondrial dysfunction and its impact on cellular energy metabolism has emerged as a critical factor in ADHD. Mitochondria, which are the primary source of adenosine triphosphate, play a pivotal role in maintaining cellular health and energy production, Interestingly, results from recent studies suggest that mitochondrial dysfunction in brain cells plays a key role in the pathogenesis of ADHD (Stefano, 2021). Dysfunctional mitochondria disrupt neuronal activity and neurotransmitter release and contribute to cognitive and behavioral symptoms associated with ADHD (Bonvento & Bolaños, 2021).
Emerging research highlights the link between mitochondrial genetics and ADHD, with variations in both nuclear and mitochondrial DNA contributing to the disorder (Giannoulis et al., 2022). Chronic activation of the cell danger response – a protective cellular mechanism triggered by infections, toxins, and/or stressors – can lead to systemic inflammation and sustained mitochondrial dysfunction (Naviaux, 2014). Such disruptions have long-term implications for neuronal plasticity and attention regulation.
Dietary habits can have a significant impact on mitochondrial function. Imbalances in essential nutrients, most notably omega-3 fatty acids and antioxidants, impair mitochondrial dynamics and exacerbate oxidative stress (Bordoni et al., 2022). Furthermore, excessive production of reactive oxygen species coupled with inadequate antioxidant defenses contributes to DNA damage and neuronal dysfunction. Supplementation with mitochondrial-supportive nutrients, for example, coenzyme Q10, mitoquinone, magnesium, and omega-3 fatty acids has been shown to have some potential to improve mitochondrial function and mitigate ADHD symptoms (Maugeri & Barchitta, 2020).
In astrocytes, mitochondrial dysfunction can limit the supply of energy required by the neurons with a negative impact on the prefrontal cortex and hippocampus. These disruptions hinder cognitive processes, including attention, working memory, and impulse control. Addressing mitochondrial health through dietary and pharmacological interventions could provide a novel approach to the management of ADHD.
5. Conclusions
ADHD continues to be a topic of interest due to its devastating and often overlooked effects on health and well-being. Topics for future research include the identification and validation of biomarkers for assessing ADHD, including inflammatory markers and daily variations in cortisol levels. One other important area of interest is the contribution of the ECS to the pathogenesis of mental health disorders. It is also important to recognize that the dietary imbalances observed among those with ADHD, notably, diets that are comparatively low in omega-3 and omega-9 fatty acids, can in and of themselves lead cause imbalances in the ECS. A healthy ECS requires fatty acids; however, Western diets involving the consumption of processed foods with very high levels of omega-6 fatty acids can lead to a pro-inflammatory state due to increased levels of arachidonic acid (Innes & Calder, 2018). The public in general has become increasingly aware of the importance of healthy foods such as those included in the Mediterranean-style diet in regulating inflammation as well as providing the healthy fats needed for overall health and optimal brain function. With respect to ADHD, supplementing psycho-pharmacological treatment and psychotherapy with critical dietary adjustments and access to nutritional supplements could lead to a profound difference for these individuals. More research is needed to explore the combined effects of traditional treatments with dietary supplementation. Furthermore, validated biomarkers might lead to a more holistic diagnostic process and could have a role in guiding the dietary habits of children and adults with ADHD.
Author Contributions: Conceptualization, S.W.; investigation, S.W.; writing—review and editing, S.W; All authors have read and agreed to this version of the manuscript.
Funding: This research received no external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: No data was used in this commentary article.
Acknowledgments: Pascal Büttiker, a Ph.D. Candidate, provided the figure, in the Department of Psychiatry, First Faculty of Medicine, Charles University in Prague, Czech Republic.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Alam, R., Abdolmaleky, H. M., & Zhou, J. R. (2017). Microbiome, inflammation, epigenetic alterations, and mental diseases. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 174(6), 651–660. https://doi.org/10.1002/ajmg.b.32567.
Aldini, G., Altomare, A., Baron, G., Vistoli, G., Carini, M., Borsani, L., & Sergio, F. (2018). N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radical Research, 52(7), 751–762. https://doi.org/10.1080/10715762.2018.1468564.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.).
Anka, A. U., Tahir, M. I., Abubakar, S. D., Alsabbagh, M., Zian, Z., Hamedifar, H., Sabzevari, A., & Azizi, G. (2021). Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis, and management. Scandinavian Journal of Immunology, 93(4), e12998. https://doi.org/10.1111/sji.12998.
Bloch, M. H., & Mulqueen, J. M. (2014). Nutritional supplements for the treatment of ADHD. Child and Adolescent Psychiatric Clinics of North America, 23(4), 883–897. https://doi.org/10.1016/j.chc.2014.05.002.
Bonvento, G., & Bolaños, J. P. (2021). Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metabolism, 33(8), 1546–1564. https://doi.org/10.1016/j.cmet.2021.07.006.
Bordoni, L., Petracci, I., Mlodzik-Czyzewska, M., Malinowska, A. M., Szwengiel, A., Sadowski, M., Gabbianelli, R., & Chmurzynska, A. (2022). Mitochondrial DNA and epigenetics: Investigating interactions with the one-carbon metabolism in obesity. Oxidative Medicine and Cellular Longevity, 2022, 9171684. https://doi.org/10.1155/2022/9171684.
Büttiker, P., Boukherissa, A., Weissenberger, S., Ptáček, R., Anders, M., Raboch, J., & Stefano, G. B. (2024). Cognitive impact of neurotropic pathogens: Investigating molecular mimicry through computational methods. Cellular and Molecular Neurobiology, 44(1), 72. https://doi.org/10.1007/s10571-024-01509-x.
Chang, J. P.-C., Mondelli, V., Satyanarayanan, S. K., Chiang, Y. J., Chen, H. T., Su, K. P., & Pariante, C. M. (2020). Cortisol, inflammatory biomarkers, and neurotrophins in children with ADHD in Taiwan. Brain, Behavior, and Immunity, 88, 105–113. https://doi.org/10.1016/j.bbi.2020.05.017.
Deepmala, Slattery, J., Kumar, N., Delhey, L., Berk, M., Dean, O. Spielholz, C., & Frye, R. (2015). N-Acetylcysteine in psychiatry and neurology: A systematic review. Neuroscience and Biobehavioral Reviews, 55, 294–317. https://doi.org/10.1016/j.neubiorev.2015.04.015.
Dunn, G. A., Nigg, J. T., & Sullivan, E. L. (2019). Neuroinflammation as a risk factor for ADHD. Pharmacology, Biochemistry, and Behavior, 182, 22–34. https://doi.org/10.1016/j.pbb.2019.05.005.
Esch, T., & Stefano, G. B. (2002). Proinflammation: A common denominator or initiator of different pathophysiological disease processes. Medical Science Monitor, 8(5), HY1–HY9.
Esch, T., Stefano, G. B., Fricchione, G. L., & Benson, H. (2002). The role of stress in neurodegenerative diseases and mental disorders. Neuroendocrinology Letters, 23(3), 199–208.
Giannoulis, S. V., Müller D., Kennedy, J. L., & Gonçalves V. (2022). Systematic review of mitochondrial genetic variation in ADHD. European Child & Adolescent Psychiatry. https://doi.org/10.1007/s00787-022-02030-6.
Han, V. X., Patel, S., Jones, H. F., & Dale, R. C. (2021). Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nature Reviews Neurology, 17(9), 564–579.
Innes, J. K., & Calder, P. C. (2018). Omega-6 fatty acids and inflammation. Prostaglandins, leukotrienes, and essential fatty acids, 132, 41–48. https://doi.org/10.1016/j.plefa.2018.03.004.
Khan, R., Naveed, S., Mian, N., Fida, A., Raafey, M. A., & Aedma, K. K. (2020). The therapeutic role of cannabidiol in mental health: A systematic review. Journal of Cannabis Research, 2(1), 2.
Khazdouz, M., Safarzadeh, R., Hejrani, B., Hasani, M., Mahdavi, F. S., Ejtahed, H. S., & Qorbani, M. (2024). The association between junk foods consumption and attention deficit hyperactivity disorder in children and adolescents: a systematic review and meta-analysis of observational studies. European child & adolescent psychiatry, 10.1007/s00787-024-02521-8. Advance online publication.
Kim, J. H., Kim, J. Y., Lee, J., Jeong, G. H., Lee, E., Lee, S., Lee, K. H., Kronbichler, A., Stubbs, B., Solmi, M., Koyanagi, A., Hong, S. H., Dragioti, E., Jacob, L., Brunoni, A. R., Carvalho, A. F., Radua, J., Thompson, T., Smith, L., Oh, H., … Fusar-Poli, P. (2020). Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. The lancet. Psychiatry, 7(11), 955–970.
Konikowska, K., Regulska-Ilow, B., & Rózańska, D. (2012). The influence of components of diet on symptoms of ADHD in children. Roczniki Panstwowego Zakladu Higieny, 63(2), 127–134.
Lopez-Leon, S., Wegman-Ostrosky, T., Perelman, C., Sepulveda, R., Rebolledo, P. A., Cuapio, A., & Villapol, S. (2021). More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Scientific reports, 11(1), 16144. https://doi.org/10.1038/s41598-021-95565-8.
Maugeri, A., & Barchitta, M. (2020). How dietary factors affect DNA methylation. Medicina (Kaunas), 56(8), 374. https://doi.org/10.3390/medicina56080374.
Navarrete, F., García-Gutiérrez, M. S., Jurado-Barba, R., Rubio, G., Gasparyan, A., Austrich-Olivares, A., & Manzanares, J. (2020). Endocannabinoid System Components as Potential Biomarkers in Psychiatry. Frontiers in psychiatry, 11, 315. https://doi.org/10.3389/fpsyt.2020.00315https://doi.org/10.3389/fpsyt.2020.00315.
Naviaux, R. K. (2014). Metabolic features of the cell danger response. Mitochondrion, 16, 7–17. https://doi.org/10.1016/j.mito.2013.08.006.
Nigg, J. T. (2013). Attention-deficit/hyperactivity disorder and adverse health outcomes. Clinical Psychology Review, 33(2), 215–228. https://doi.org/10.1016/j.cpr.2012.11.005.
Oades, R. D., Dauvermann, M.R., Schimmelmann, B.G., Schwarz, M.J., & Myint, A.-M. (2010). Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism - effects of medication. Behavioral and Brain Functions, 6(1), 29. https://doi.org/10.1186/1744-9081-6-29.
Park, J.H. (2022). Potential Inflammatory Biomarker in Patients with Attention Deficit Hyperactivity Disorder. Int. J. Mol. Sci., 23, 13054. https://doi.org/10.3390/ijms232113054.
Polanczyk, G., de Lima, M. S., Horta, B. L., Biederman, J., & Rohde, L. A. (2007). The Worldwide Prevalence of ADHD: A Systematic Review and Metaregression Analysis. American Journal of Psychiatry, 164(6), 942–948. https://doi.org/10.1176/ajp.2007.164.6.942.
Rios-Hernandez, A., Alda, J.A., Farran-Codina, A., Ferreira-García, E., & Izquierdo-Pulido, M. (2017). The Mediterranean Diet and ADHD in Children and Adolescents. Pediatrics, 139(2), e20162027. https://doi.org/10.1542/peds.2016-2027.
Sanchez, C. E., Barry, C., Sabhlok, A., Russell, K., Majors, A., Kollins, S. H., & Fuemmeler, B. F. (2018). Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obesity Reviews, 19(4), 464–484. https://doi.org/10.1111/obr.12643.
Stefano, G. B. (2021). Historical insight into infections and disorders associated with neurological and psychiatric sequelae similar to long COVID. Medical Science Monitor 27, e931447, https://doi.org/10.12659/MSM.931447.
Stefano, G. B. (2024). Viral targeting of mitochondria may alter cognition and enhance viral transmission. The Mind - Bulletin on Mind-Body Medicine Research 2, https://doi.org/10.61936/themind/202406043.
Stefano, G. B., Pilonis, N., Ptáček, R., Raboch, J., Vnukova, M., & Kream, R. M. (2018). Gut, microbiome, and brain regulatory axis: Relevance to neurodegenerative and psychiatric disorders. Cellular and Molecular Neurobiology, 38(6), 1197–1206. https://doi.org/10.1007/s10571-018-0589-2.
Stefano, G. B., Weissenberger, S., Ptáček, R., Anders, M., Raboch, J., & Büttiker, P. (2024). Viruses and mitochondrial dysfunction in neurodegeneration and cognition: An evolutionary perspective. Cellular and Molecular Neurobiology, 44(1), 68. https://doi.org/10.1007/s10571-024-01503-3.
Tabatadze, T., Kherkheulidze, M., Kandelaki, E., Kavlashvili, N., & Ivanashvili, T. (2018). ATTENTION DEFICIT HYPERACTIVITY DISORDER AND HAIR HEAVY METAL AND ESSENTIAL TRACE ELEMENT CONCENTRATIONS. IS THERE A LINK?. Georgian medical news, (284), 88–92.
Varlaet, C., Maasakkers, C., Hermans, N., & Savelkoul, H. (2018). Rationale for dietary antioxidant treatment of ADHD. Nutrients, 10(4), 405. https://doi.org/10.3390/nu10040405.
Wang, S., & Qi, X. (2022). The putative role of astaxanthin in neuroinflammation modulation: Mechanisms and therapeutic potential. Frontiers in Pharmacology, 13, 916653. https://doi.org/10.3389/fphar.2022.916653.
Weissenberger, S., Ptacek, R., Klicperova-Baker, M., Erman, A., Schonova, K., Raboch, J., & Goetz M. (2017). ADHD, lifestyles and comorbidities: a call for an holistic perspective - from medical to societal intervening factors. Frontiers in Psychology, 8, 454. https://doi.org/10.3389/fpsyg.2017.00454.
Weissenberger, S., Ptáček, R., Vnukova, M., Raboch, J., Klicperova-Baker, M., Domkarova, L., & Goetz, M. (2018). ADHD and lifestyle habits in Czech adults, a national sample. Neuropsychiatric Disease and Treatment, 14, 293–299. https://doi.org/10.2147/NDT.S148921.

